This website is intended only for healthcare professionals.
To access the site, please click YES if you are a healthcare professional.
If you are not a healthcare professional, select NO to exit.

Rapid and minimally invasive procedure 2
Rapid and long lasting response 2
HYADD®4 is an innovative hyaluronic acid hydrogel developed by Fidia Farmaceutici, marketed under the brand name HYMOVIS®. This advanced formulation features a unique hexadecylamide derivative of hyaluronic acid, enhancing its viscoelastic properties and prolonging its residence time in the joint


Clinical studies have demonstrated its efficacy in reducing symptoms and enhancing the quality of life for high demand patients with OA.
The hydrogel is administered via intra-articular injection, offering a promising alternative for those who have not responded well to conventional treatments.

4th generation intra-articular HA based
product MD3
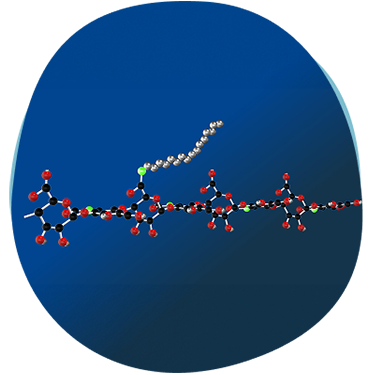
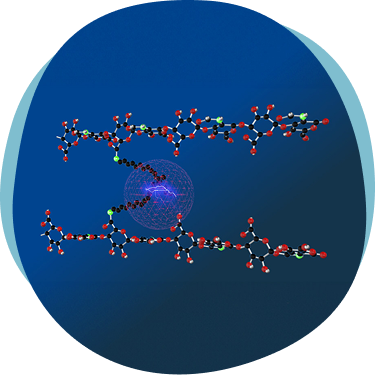
All information you are about to see is related to a specific selected market, including the products’ portfolio, and therefore comply to different local regulations.